What Is The Dri For Vitamin C
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
Summary
This report provides quantitative recommendations for the intake of vitamin C, vitamin E, and selenium. It also discusses β-carotene and other carotenoids (α-carotene, β-cryptoxanthin, lutein, lycopene, and zeaxanthin) but does not provide quantitative recommendations for their intake. It is one volume in a series of reports that presents dietary reference values for the intake of nutrients by Americans and Canadians. The development of Dietary Reference Intakes (DRIs) expands and replaces the series of Recommended Dietary Allowances (RDAs) in the United States and Recommended Nutrient Intakes (RNIs) in Canada. The report includes current concepts about the roles vitamin C, vitamin E, selenium, and β-carotene and the other carotenoids play in long-term health, going beyond a review of the roles they are known to play in traditional deficiency diseases. A major impetus for the expansion of this review is the growing recognition of the many uses to which RDAs and RNIs have been applied, and a growing awareness that many of these uses require the application of statistically valid methods that depend on reference values other than recommended nutrient intakes.
The overall project is a comprehensive effort undertaken by the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (DRI Committee) of the Food and Nutrition Board, Institute of Medicine, the National Academies, with active involvement of Health Canada. (See Appendix A for a description of the overall process and its origins.) This study was requested by the Federal Project Steering Committee for Dietary Reference Intakes
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
(see Appendix B for membership), which is coordinated by the Office of Disease Prevention and Health Promotion of the U.S. Department of Health and Human Services, in collaboration with Health Canada.
Major new recommendations in this report include the following:
-
A definition of a dietary antioxidant is provided.
-
The Recommended Dietary Allowance (RDA) for vitamin E and selenium is the same for adult men and women regardless of age, representing the lack of specificity in data available.
-
The Recommended Dietary Allowance (RDA) for vitamin C is different for adult men and women due to women's smaller lean body mass.
-
α-Tocopherol alone is used for estimating vitamin E requirements and recommending daily vitamin E intake, since the other naturally occurring forms of vitamin E (β-, γ-, and δ-tocopherols and the tocotrienols) are not converted to α-tocopherol in the human and are recognized poorly by the α-tocopherol transfer protein in the liver.
-
Tolerable Upper Intake Levels (ULs) for vitamin C, vitamin E, and selenium are established.
-
Research recommendations for full-scale intervention trials to test the preventive potential of vitamin C, vitamin E, selenium, and β-carotene and other carotenoids for chronic disease are outlined. At the present time, there is no resolution of the possible impact of these nutrients or food components on chronic disease.
WHAT ARE DIETARY REFERENCE INTAKES?
Dietary Reference Intakes (DRIs) are reference values that are quantitative estimates of nutrient intakes to be used for planning and assessing diets for apparently healthy people. They include Recommended Dietary Allowances (RDAs) as well as three other types of reference values (see Box S-1). Although the reference values are based on published data, the data were often scanty or drawn from studies that had limitations in addressing the question. Thus, scientific judgment was required for evaluating the evidence and in setting the reference values and is delineated for each nutrient in Chapter 5, Chapter 6, Chapter 7 through Chapter 8.
Recommended Dietary Allowances
The process for setting the RDA depends on being able to set an Estimated Average Requirement (EAR). Before setting the EAR, a spe-
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
cific criterion of adequacy is selected, based on a careful review of the literature. When selecting the criterion, reduction of disease risk is considered along with many other health parameters.
Box S-1 Dietary Reference Intakes
Recommended Dietary Allowance (RDA): the dietary intake level that is sufficient to meet the nutrient requirement of nearly all (97 to 98 percent) healthy individuals in a particular life stage and gender group.
Adequate Intake (AI): a recommended intake value based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of healthy people that are assumed to be adequate—used when an RDA cannot be determined.
Tolerable Upper Intake Level (UL): the highest level of nutrient intake that is likely to pose no risk of adverse health effects for almost all individuals in the general population. As intake increases above the UL, the risk of adverse effects increases.
Estimated Average Requirement (EAR): a nutrient intake value that is estimated to meet the requirement of half the healthy individuals in a life stage and gender group.
If the standard deviation (SD) of the EAR is available and the requirement for the nutrient is symmetrically distributed, the RDA is set at 2 SDs above the EAR:
RDA = EAR + 2 SD EAR .
If data about variability in requirements are insufficient to calculate an SD, a coefficient of variation (CV) for the EAR of 10 percent is ordinarily assumed, unless available data indicate a greater variation is probable.
If 10 percent is assumed to be the CV, then twice that amount added to the EAR is defined as equal to the RDA. The resulting equation for the RDA is then
RDA = 1.2 × EAR.
If the distribution of the nutrient requirement is known to be skewed for a population, other approaches are used to find the ninety-seventh to ninety-eighth percentile to set the RDA. The RDA for a nutrient is a value to be used as a goal for dietary intake for the healthy individual. As discussed in Chapter 9 of this report, the
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
RDA is not intended to be used to assess the diets of either individuals or groups or to plan diets for groups.
Adequate Intakes
The Adequate Intake (AI) is set instead of an RDA if sufficient scientific evidence is not available to calculate an EAR. For example, the AI for young infants, for whom human milk is the recommended sole source of food for most nutrients up through the first 4 to 6 months, is based on the daily mean nutrient intake of apparently healthy, full-term infants who receive only human milk. The main intended use of the AI is as a goal for the nutrient intake of individuals. Other uses of AIs will be considered in future reports.
Comparison of RDAs and AIs
Although both the RDA and the AI are to be used as a goal for intake by individuals, the RDA differs from the AI. Intake of the RDA for a nutrient is expected to meet the needs of 97 to 98 percent of the individuals in a life stage and gender group. However, because no distribution of requirements is known for nutrients with an AI, it is not possible to know what percentage of individuals are covered by the AI. In determining the AI for a nutrient, it is expected to exceed the RDA for that nutrient, if it were known, and should cover the needs of more than 97 to 98 percent of the individuals (see Figure S-1). The degree to which an AI exceeds the RDA is likely to differ among nutrients and population groups, however.
For people with diseases that increase specific nutrient requirements or those who have other special health needs, the RDA and AI may each serve as the basis for adjusting individual recommendations; qualified health professionals should adapt the recommended intake to cover higher or lower needs.
Table S-1, Table S-2 through Table S-3 give the recommended intake levels, whether RDAs or AIs, for vitamin C, vitamin E (α-tocopherol), and selenium by life stage and gender group. For these nutrients, AIs rather than RDAs are being proposed for infants to age 1 year.
Tolerable Upper Intake Levels
The Tolerable Upper Intake Level (UL) is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects for almost all individuals in the general population. As intake increases above the UL, the risk of adverse effects increases. The term
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
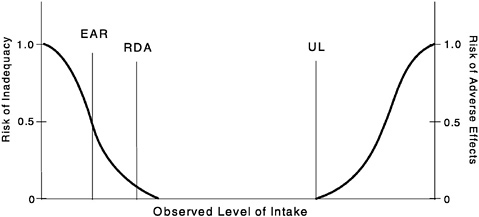
FIGURE S-1 Dietary reference intakes. This figure shows that the Estimated Average Requirement (EAR) is the intake at which the risk of inadequacy is 0.5 (50%) to an individual. The Recommended Dietary Allowance (RDA) is the intake at which the risk of inadequacy is very small —only 0.02 to 0.03 (2% to 3%). The Adequate Intake (AI) does not bear a consistent relationship to the EAR or the RDA because it is set without being able to estimate the average requirement. It is assumed that the AI is at or above the RDA if one could be calculated. At intakes between the RDA and the Tolerable Upper Intake Level (UL), the risks of inadequacy and of excess are both close to 0. At intakes above the UL, the risk of adverse effects may increase.
"tolerable intake" was chosen to avoid implying a possible beneficial effect. Instead, the term is intended to connote a level of intake that can, with high probability, be tolerated biologically. The UL is not intended to be a recommended level of intake. There is no established benefit for apparently healthy individuals if they consume nutrient intakes above the RDA or AI.
ULs are useful because of the increased interest in and availability of fortified foods and the increased use of dietary supplements. ULs are based on total intake of a nutrient from food, water, and supplements if adverse effects have been associated with total intake. However, if adverse effects have been associated with intake from supplements or food fortificants only, the UL is based on nutrient intake from these sources only, rather than on total intake. The UL applies to chronic daily use.
For some nutrients such as β-carotene and other carotenoids, there are insufficient data with which to develop a UL. This does
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
TABLE S-1 Criteria and Dietary Reference Intake Values for Vitamin C by Life Stage and Gender Group
| Life Stage Group | Criterion |
| 0 through 6 mo | Human milk content |
| 7 through 12 mo | Human milk + solid food |
| 1 through 3 y | Extrapolation from adult |
| 4 through 8 y | Extrapolation from adult |
| 9 through 13 y | Extrapolation from adult |
| 14 through 18 y | Extrapolation from adult |
| 19 through 30 y | Near-maximal neutrophil concentration |
| 31 through 50 y | Extrapolation of near-maximal neutrophil concentration from 19 through 30 y |
| 51 through 70 y | Extrapolation of near-maximal neutrophil concentration from 19 through 30 y |
| >70 y | Extrapolation of near-maximal neutrophil concentration from 19 through 30 y |
| Pregnancy | |
| ≤18 y | Extrapolation of near-maximal neutrophil concentration plus transfer to the fetus |
| 19 through 50 y | Extrapolation of near-maximal neutrophil concentration plus transfer to the fetus |
| Lactation | |
| ≤18 y | Human milk content + age specific requirement |
| 19 through 50 y | Human milk content + age specific requirement |
| a EAR = Estimated Average Requirement. The intake that meets the estimated nutrient needs of half of the individuals in a group. b RDA = Recommended Dietary Allowance. The intake that meets the nutrient needs of almost all (97–98 percent) individuals in a group. c AI = Adequate Intake. The observed average or experimentally set intake by a defined population or subgroup that appears to sustain a defined nutritional status, such as growth rate, normal circulating nutrient values, or other functional indicators of health. An AI is used if sufficient scientific evidence is not available to derive an EAR. For healthy human milk-fed infants, the AI is the mean intake. The AI is not equivalent to an RDA. | |
not mean that there is no potential for adverse effects resulting from high intake. When data about adverse effects are extremely limited, extra caution may be warranted.
APPROACH FOR SETTING DIETARY REFERENCE INTAKES
The scientific data used to develop Dietary Reference Intakes (DRIs) have come from observational and experimental studies. Studies published in peer-reviewed journals were the principal
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
| EAR (mg/d)a | RDA (mg/d)b | AI (mg/d)c | |||
| Male | Female | Male | Female | Male | Female |
| 40 | 40 | ||||
| 50 | 50 | ||||
| 13 | 13 | 15 | 15 | ||
| 22 | 22 | 25 | 25 | ||
| 39 | 39 | 45 | 45 | ||
| 63 | 56 | 75 | 65 | ||
| 75 | 60 | 90 | 75 | ||
| 75 | 60 | 90 | 75 | ||
| 75 | 60 | 90 | 75 | ||
| 75 | 60 | 90 | 75 | ||
| 66 | 80 | ||||
| 70 | 85 | ||||
| 96 | 115 | ||||
| 100 | 120 | ||||
source of data. Life stage and gender were considered to the extent possible, but the data did not provide a basis for proposing different requirements for men and for nonpregnant and nonlactating women in different age groups for any of the nutrients except vitamin C.
Three of the categories of reference values (Estimated Average Requirement [EAR], Recommended Dietary Allowance [RDA], and Adequate Intake [AI]) are defined by specific criteria of nutrient adequacy; the fourth (Tolerable Upper Intake Level [UL]) is defined by a specific endpoint of adverse effect, when one is avail-
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
TABLE S-2 Criteria and Dietary Reference Intake Values for α-Tocopherol a by Life Stage Group
| Life Stage Group b | Criterion |
| 0 through 6 mo | Human milk content |
| 7 through 12 mo | Extrapolation from 0 to 6 mo |
| 1 through 3 y | Extrapolation from adult |
| 4 through 8 y | Extrapolation from adult |
| 9 through 13 y | Extrapolation from adult |
| 14 through 18 y | Extrapolation from adult |
| 19 through 30 y | Prevention of hydrogen peroxide-induced hemolysis |
| 31 through 50 y | Extrapolation of hydrogen peroxide-induced hemolysis from 19 through 30 y |
| 51 through 70 y | Extrapolation of hydrogen peroxide-induced hemolysis from 19 through 30 y |
| >70 y | Extrapolation of hydrogen peroxide-induced hemolysis from 19 through 50 y |
| Pregnancy | |
| ≤18 y | Plasma concentration |
| 19 through 50 y | Plasma concentration |
| Lactation | |
| ≤18 y | Human milk content + age specific requirement |
| 19 through 50 y | Human milk content + age specific requirement |
| a α-Tocopherol includes RRR-α-tocopherol, the only form of α-tocopherol that occurs naturally in foods, and the 2R-stereoisomeric forms of α-tocopherol (RRR-, RSR-, RRS-, and RSS-α-tocopherol) that occur in fortified foods and supplements. Does not include the 2S-stereoisomeric forms of α-tocopherol (SRR-, SSR-, SRS-, and SSS-α-tocopherol), also found in fortified foods and supplements. The 2R-stereoisomeric forms of α-tocopherol, as defined in this report, are the only forms of Vitamin E that have been shown to meet human requirements. b All groups except Pregnancy and Lactation are males and females. c EAR = Estimated Average Requirement. The intake that meets the estimated nutrient needs of half of the individuals in a group, men and women combined. | |
able. In all cases, data were examined closely to determine whether an antioxidant function or a reduction of risk of a chronic degenerative disease could be used as a criterion of adequacy. The quality of studies was examined by considering study design; methods used for measuring intake and indicators of adequacy; and biases, interactions, and confounding factors.
Although the reference values are based on data, the data were often scanty or drawn from studies that had limitations in addressing the various questions that confronted the panel. Therefore,
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
| EAR (mg/d) c | RDA (mg/d) d | AI (mg/d) e |
| 4 | ||
| 6 | ||
| 5 | 6 | |
| 6 | 7 | |
| 9 | 11 | |
| 12 | 15 | |
| 12 | 15 | |
| 12 | 15 | |
| 12 | 15 | |
| 12 | 15 | |
| 12 | 15 | |
| 12 | 15 | |
| 16 | 19 | |
| 16 | 19 | |
d RDA = Recommended Dietary Allowance. The intake that meets the nutrient needs of almost all (97–98 percent) individuals in a group.
e AI = Adequate Intake. The observed average or experimentally set intake by a defined population or subgroup that appears to sustain a defined nutritional status, such as growth rate, normal circulating nutrient values, or other functional indicators of health. An AI is used if sufficient scientific evidence is not available to derive an EAR. For healthy human milk-fed infants, the AI is the mean intake. The AI is not equivalent to an RDA.
many of the questions raised about the requirements for and recommended intakes of these nutrients cannot be answered fully because of inadequacies in the present database. Apart from studies of overt deficiency diseases, there is a dearth of studies that address specific effects of inadequate intakes on specific indicators of health status. (A research agenda is proposed; see Chapter 10.) After careful review and analysis of the evidence, including examination of the extent of congruence of findings, scientific judgment was used to determine the basis for establishing the values. The reasoning
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
TABLE S-3 Criteria and Dietary Reference Intake Values for Selenium by Life Stage Group
| Life Stage Group a | Criterion |
| 0 through 6 mo | Human milk content |
| 7 through 12 mo | Human milk + solid food |
| 1 through 3 y | Extrapolation from adult |
| 4 through 8 y | Extrapolation from adult |
| 9 through 13 y | Extrapolation from adult |
| 14 through 18 y | Extrapolation from adult |
| 19 through 30 y | Maximizing plasma glutathione peroxidase activity |
| 31 through 50 y | Extrapolation of plasma glutathione peroxidase activity from 19 through 30 y |
| 51 through 70 y | Extrapolation of plasma glutathione peroxidase activity from 19 through 30 y |
| >70 y | Extrapolation of plasma glutathione peroxidase activity from 19 through 30 y |
| Pregnancy | |
| ≤18 y | Saturation of fetal selenoprotein |
| 19 through 50 y | Saturation of fetal selenoprotein |
| Lactation | |
| ≤18 y | Human milk content + age specific requirement |
| 19 through 50 y | Human milk content + age specific requirement |
| a All groups except Pregnancy and Lactation are males and females. b EAR = Estimated Average Requirement. The intake that meets the estimated nutrient needs of half of the individuals in a group, men and women combined. c RDA = Recommended Dietary Allowance. The intake that meets the nutrient needs of almost all (97–98 percent) individuals in a group. d AI = Adequate Intake. The observed average or experimentally set intake by a defined population or subgroup that appears to sustain a defined nutritional status, such as growth rate, normal circulating nutrient values, or other functional indicators of health. An AI is used if sufficient scientific evidence is not available to derive an EAR. For healthy human milk-fed infants, the AI is the mean intake. The AI is not equivalent to an RDA. | |
used is described for each nutrient in Chapters 5 through Chapter 8. While the various recommendations are provided as single rounded numbers for practical considerations, it is acknowledged that these values imply a precision not fully justified by the underlying data in the case of currently available human studies.
In this report, the scientific evidence related to the prevention of chronic degenerative disease was judged to be too nonspecific to be used as the basis for setting any of the recommended levels of intake. Furthermore, a quantitative relationship between the biomarkers of antioxidant function and the prevention of chronic
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
| EAR (µg/d) b | RDA (µg/d) c | AI (µg/d) d |
| 15 | ||
| 20 | ||
| 17 | 20 | |
| 23 | 30 | |
| 35 | 40 | |
| 45 | 55 | |
| 45 | 55 | |
| 45 | 55 | |
| 45 | 55 | |
| 45 | 55 | |
| 49 | 60 | |
| 49 | 60 | |
| 59 | 70 | |
| 59 | 70 | |
degenerative disease was lacking. Thus, for vitamin C, vitamin E, and selenium, EARs and RDAs are based on criteria specifically related to their general functions. For all of these nutrients, the EAR is higher than the amount needed to prevent overt deficiency diseases in essentially all individuals in the life stage group and is based on limited data indicating laboratory evidence of sufficiency. At this time, no DRIs have been set for any of the carotenoids. The indicators used in deriving the EARs and thus the RDAs are described below.
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
NUTRIENT FUNCTIONS AND THE INDICATORS USED TO ESTIMATE REQUIREMENTS FOR VITAMIN C, VITAMIN E, SELENIUM, AND THE CAROTENOIDS
Vitamin C (ascorbic acid) functions physiologically as a water-soluble antioxidant by virtue of its high reducing power. To provide antioxidant protection, the Recommended Dietary Allowance (RDA) for adults for vitamin C is set at 75 mg/day for females and 90 mg/day for males. This intake should maintain near maximal neutrophil ascorbate concentrations with little urinary excretion. Because smokers suffer increased oxidative stress and metabolic turnover of vitamin C, their recommended intake is increased by 35 mg/day.
Vitamin E is thought to function primarily as a chain-breaking antioxidant that prevents the propagation of lipid peroxidation. To estimate the requirement, data were examined on induced vitamin E deficiency in humans and the intake that correlated with in vitro hydrogen peroxide-induced hemolysis and plasma α-tocopherol concentrations. In addition, vitamin E acts as an in vivo antioxidant, maintaining normal physiological function in humans. The RDA for both men and women is 15 mg/day of α-tocopherol. Other naturally occurring forms of vitamin E (β-, γ-, δ-tocopherol and the tocotrienols) do not meet the vitamin E requirement because they are not converted to α-tocopherol in humans and are recognized poorly by the α-tocopherol transfer protein. In establishing recommended intakes α-tocopherol is defined as RRR-α-tocopherol, the only form of α-tocopherol that occurs naturally in food, and the 2R-stereoisomeric forms of α-tocopherol (RRR-, RSR-, RRS-, and RSS-α-tocopherol) that occur in fortified foods and supplements.
Selenium functions through selenoproteins, several of which are oxidant defense enzymes. The method used to estimate the requirement for selenium relates to the intake needed to maximize the activity of the plasma selenoprotein glutathione peroxidase, an oxidant defense enzyme. The RDA for both men and women is 55 µg/day. It is not clear if the diseases associated with selenium deficiencies, Keshan disease or Kashin-Beck disease, are due to oxidative stress. The selenium in several selenoproteins has a biochemical role in oxidant defense, and as such plays a role as a dietary antioxidant.
β-Carotene and other provitamin A carotenoids function as a source of vitamin A and, due to this provitamin A activity, can prevent vitamin A deficiency. Because specific functions beyond this role have not yet been sufficiently identified, no Dietary Reference Intakes (DRIs)
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
have been established for any of the carotenoids including those which do not have provitamin A activity. In conjunction with the review of vitamin A, efforts are under way to establish ratios for the provitamin A carotenoids—β-carotene, α-carotene, and β-cryptoxanthin—based on their ability to be converted to vitamin A. A subsequent report will provide this analysis of the potential contributions of the carotenoids to the requirement for vitamin A.
CRITERIA AND PROPOSED VALUES FOR TOLERABLE UPPER INTAKE LEVELS
A risk assessment model is used to derive Tolerable Upper Intake Levels (ULs). The model consists of a systematic series of scientific considerations and judgments. The hallmark of the risk assessment model is the requirement to be explicit in all the evaluations and judgments made.
The ULs for adults for vitamin C (2,000 mg/day based on the adverse effect of osmotic diarrhea), vitamin E (1,000 mg/day of any form of supplemental α-tocopherol based on the adverse effect of increased tendency to hemorrhage), and selenium (400 µg/day based on the adverse effect of selenosis), shown in Table S-4, were set to protect the most sensitive individuals in the general population (e.g., those who might be below reference adult weight). Members of the general apparently healthy population should be advised not to exceed the UL routinely. However, intake above the UL may be appropriate for investigation within well-controlled clinical trials to ascertain if such intakes are of benefit to health. Clinical trials of doses above the UL should not be discouraged because it is expected that participation in these trials will require informed consent that will include discussion of the possibility of adverse effects and will employ appropriate safety monitoring of trial subjects.
The ULs for vitamin C and selenium are based on intake from diet and supplements. Vitamin E ULs are based on intake from supplements only.
A UL could not be established for β-carotene because of inconsistent data and could not be set for other carotenoids because of a lack of suitable data. In both cases, this signifies a need for additional information. It does not necessarily signify that people can tolerate chronic intakes of these substances at high levels. Like all chemical agents, nutrients and other food components can produce adverse effects if intakes are excessive. Therefore, when data are extremely limited, extra caution may be warranted. In particular, β-carotene supplementation is not advisable, other than for the
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
TABLE S-4 Tolerable Upper Intake Levels (UL a ) by Life Stage Group
| Life Stage Group | Vitamin C (mg/d) | α-Tocopherol (mg/d) b | Selenium (µg/d) |
| 0 through 6 mo | ND c | ND | 45 |
| 7 through 12 mo | ND | ND | 60 |
| 1 through 3 y | 400 | 200 | 90 |
| 4 through 8 y | 650 | 300 | 150 |
| 9 through 13 y | 1,200 | 600 | 280 |
| 14 through 18 y | 1,800 | 800 | 400 |
| 19 through 70 y | 2,000 | 1,000 | 400 |
| >70 y | 2,000 | 1,000 | 400 |
| Pregnancy | |||
| ≤18 y | 1,800 | 800 | 400 |
| 19 through 50 y | 2,000 | 1,000 | 400 |
| Lactation | |||
| ≤18 y | 1,800 | 800 | 400 |
| 19 through 50 y | 2,000 | 1,000 | 400 |
| a The UL is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population. As intake increases above the UL, the risk of adverse effects increases. Unless specified otherwise, the UL represents total nutrient intake from food, water, and supplements. b The UL for α-tocopherol applies to any form of supplemental α-tocopherol. c ND. Not determinable due to lack of data of adverse effects in this age group and concern with regard to lack of ability to handle excess amounts. Source of intake should be from food and formula in order to prevent high levels of intake. | |||
prevention and control of vitamin A deficiency, in view of concerns about lung cancer and total mortality risk raised by recent randomized clinical trials in special at-risk populations.
USING DIETARY REFERENCE INTAKES
Suggested uses of Dietary Reference Intakes (DRIs) appear in Box S-2. The transition from using previously published Recommended Dietary Allowance (RDAs) and Reference Nutrient Intakes (RNIs) alone to using all DRIs appropriately will require time and effort by health professionals and others.
For statistical reasons that will be addressed in a future report and discussed briefly in Chapter 9, the Estimated Average Requirement (EAR) is the appropriate reference intake to use in assessing the
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
nutrient intake of groups; the RDA is not appropriate. The prevalence of inadequacy may be estimated by determining the percentage of the population below the EAR as follows:
-
Based on the Third National Health and Nutrition Examination Survey (NHANES III) data, about 11 percent of nonsmoking
Box S-2 Uses of Dietary Reference Intakes for Healthy Individuals and Groups
| Type of Use | For the Individual | For a Group |
| Assessment | EAR a : use to examine the possibility of inadequacy of reported intake. | EAR b : use to estimate the prevalence of inadequate intakes within a group. |
| AI a : intakes at this level have a low probability of inadequacy. | AI b : mean intake at this level implies a low prevalence of inadequate intakes. | |
| UL a : intake above this level has a risk of adverse effects. | UL b : use to estimate the prevalence of intakes that may be at risk of adverse effects. | |
| Planning | RDA: aim for this intake. | EAR: use in conjunction with a measure of variability of the group's intake to set goals for the median intake of a specific population. |
| AI: aim for this intake. | ||
| UL: use as a guide to limit intake; chronic intake of higher amounts may increase risk of adverse effects. | ||
| EAR = Estimated Average Requirement RDA = Recommended Dietary Allowance AI = Adequate Intake UL = Tolerable Upper Intake Level a Requires accurate measure of usual intake. Evaluation of true status requires clinical, biochemical, and anthropometric data. b Requires statistically valid approximation of usual intake. | ||
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
-
adult women and 21 percent of nonsmoking adult men have dietary intakes of vitamin C that are less than the EAR for this nutrient.
-
Although dietary intakes of selenium depend on the selenium content of the soil where a plant was grown, adults in North America are meeting their selenium needs, probably because food in the United States and Canada is so widely distributed beyond the region where it was grown.
-
Only a small proportion of the adult men and women in the population reportedly has a vitamin E intake from food and supplements greater than the EAR. However, estimates of vitamin E intake are particularly difficult due to a propensity to underreport fat intake which results in its underestimation (dietary fat serves as the major carrier for vitamin E). In addition, the EARs for vitamin E are based on α-tocopherol only and do not include amounts obtained from the other seven naturally occurring forms of vitamin E (β-, γ-, δ-tocopherol and the four tocotrienols). Because the various forms of vitamin E cannot be interconverted in humans, EARs, RDAs, and AIs apply only to intake of the 2R-stereoisomeric forms of α-tocopherol from food, fortified foods, and multivitamins. Currently, most nutrient databases, as well as nutrition labels, do not distinguish among the various tocopherols in food. They often present the data as α-tocopherol equivalents and include the contribution of all eight naturally occurring forms of vitamin E, after adjustment for bioavailability (e.g., γ-tocopherol is usually assumed to have only 10 percent of the availability of α-tocopherol). Because these other forms of vitamin E occur in foods (e.g., γ-tocopherol is present in widely consumed oils such as soybean and corn oils), the intake of α-tocopherol equivalents is greater than the intake of α-tocopherol alone. Based on NHANES III dietary intake data, approximately 80 percent of the α-tocopherol equivalents from food are from α-tocopherol, and thus can contribute to the body's requirement for vitamin E.
-
Data for intakes of vitamin C, vitamin E, and selenium from food and supplements in the United States are provided in this report. Data from Canada are available only for vitamin C from food. Detailed data for intakes of carotenoids from a recently released and expanded food composition database in the United States are presently being analyzed and are not available to be included in this report. Thus they will be included in the Appendix of a subsequent DRI report that will include vitamin A.
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
DEFINITION OF A DIETARY ANTIOXIDANT
A dietary antioxidant is a substance in foods that significantly decreases the adverse effects of reactive species, such as reactive oxygen and nitrogen species, on normal physiological function in humans. The definition is based on several criteria: the substance is found in human diets, the content of the substance has been measured in foods commonly consumed, and the substance decreases the adverse effects of reactive species in vivo in humans. Vitamin C, vitamin E, and selenium (in the form of selenocysteine or selenomethionine) are the food components reviewed in this report that meet this definition of a dietary antioxidant. The other food components covered in this report, β-carotene and the other carotenoids, do not meet the definition but influence biochemical reactions that involve the oxidative process.
EVIDENCE OF OXIDATIVE STRESS AND THE RISK OF CHRONIC DEGENERATIVE DISEASE
There is a considerable body of biological evidence that, at high levels, reactive oxygen and nitrogen species can be damaging to cells and thus may contribute to cellular dysfunction and disease. Hence, close attention has been given to evidence relating intake of vitamin C, vitamin E, selenium, and β-carotene and other carotenoids to reduction of the risk of chronic disease. Since the entire population is exposed to oxidative stresses through oxidative metabolism and only some develop a chronic disease, it is clear that more information is needed in order to understand how to evaluate the role of oxidative stress in the development of chronic disease. The potential role of oxidative stress in six chronic disease relationships is briefly described below.
Cancer
One theory holds that oxidative damage contributes to carcinogenesis. A great deal of epidemiological evidence indicates that diets rich in fruits and vegetables are associated with a lower risk of incurring a number of common cancers, especially cancers of the lung, oral cavity, pharynx, larynx, and cervix. However, these studies provide only limited support for a protective association of individual food components categorized as antioxidants. Data regarding the protection by individual food components against cancer in humans are not yet available.
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
Cardiovascular Disease
Of all the chronic diseases in which excess oxidative stress has been implicated, cardiovascular disease has the strongest supporting evidence. Oxidation of low-density lipoproteins may be a key step in the development of coronary atherosclerosis. Epidemiological studies indicate that diets rich in fruits and vegetables, vitamin C, vitamin E, and carotenoids are associated with a decreased risk of coronary heart disease. However, no randomized prospective studies have documented a favorable effect of vitamin C or carotenoids on cardiovascular morbidity and mortality. Four studies have examined the effects of vitamin E; only one reported a positive benefit while the other three were neutral. Thus available data do not adequately substantiate the premise that increasing the intake of vitamin C, vitamin E, or β-carotene and other carotenoids will reduce the risk of coronary heart disease. Ongoing randomized trials among high-risk, apparently healthy individuals and among patients with cardiovascular disease are expected to provide evidence useful in resolving this issue.
Cataracts
A number of observational epidemiological studies have examined the relationship between intakes of vitamin C, vitamin E, and carotenoids and the presence of cataracts in humans. Several studies indicate a lowered risk of cataracts associated with either an increased serum level of these dietary components or supplement use. These studies, since observational in nature, do not constitute at this time a sufficient basis for a conclusion that these dietary components can prevent cataracts in humans.
Age-Related Macular Degeneration
Epidemiological studies find a decreased likelihood of age-related macular degeneration (AMD) associated with higher intakes of fruits and vegetables, especially those that are rich in the carotenoids lutein and zeaxanthin. Protective effects of lutein and zeaxanthin are biologically plausible because these carotenoids selectively accumulate as the pigment of the macular region of the retina and account for the yellow color observed in this region. The association has also been observed in smokers, who have lower plasma levels of carotenoids and are also at an increased risk of developing AMD. However, all reports are associative in nature and have not
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
established a causal relationship between intake or plasma concentrations of lutein and/or zeaxanthin and risk for AMD.
Central Neurodegenerative Diseases
Increasing evidence suggests that a number of common neurode-generative diseases, such as Alzheimer's, Parkinson's, and amyotrophic lateral sclerosis, may reflect adverse responses to oxidative stress. Small intervention trials with either vitamin C or vitamin E have reported symptomatic improvement in those already afflicted with the disease. However, these preliminary findings do not constitute adequate proof of the usefulness of these antioxidants in decreasing the development or delaying the onset of these diseases.
Diabetes Mellitus
Although some evidence suggests that modifications observed in structural proteins in patients with diabetes mellitus may be attributable to either an oxidative stress or a stress due to reactive carbonyls, much of the research, with either single compounds or combinations of specific food components that may function as antioxidants, has been inconclusive. In addition, no clinical intervention trials have tested directly whether provision of antioxidants can defer the onset of the complications of diabetes.
RECOMMENDATIONS
Available Data on Food Composition
Because the various forms of vitamin E are not interconvertible and because plasma concentrations of α-tocopherol are dependent upon the affinity of the hepatic α-tocopherol transfer protein for the various forms, it is recommended that relative biological potencies of the various forms of vitamin E be reevaluated. Until this is done, the actual concentrations of each of the various vitamin E forms in food and biological samples should be reported separately, wherever possible.
Research
Five major types of information gaps were noted: (1) a dearth of studies designed specifically to estimate average requirements in apparently healthy humans; (2) a nearly complete lack of usable
Suggested Citation:"Summary." Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. doi: 10.17226/9810.
×
data on the nutrient needs of infants, children, adolescents, and pregnant and lactating women; (3) a lack of definitive studies to determine the role of these nutrients in lowering the risk of certain chronic diseases; (4) a lack of validated biomarkers to evaluate oxidative stress and the relationship between antioxidant intake and health and disease; and (5) a lack of studies designed to detect adverse effects of chronic high intakes of these nutrients.
Highest priority is thus given to research that has potential to prevent or retard human disease processes and to prevent deficiencies with functional consequences as follows:
-
Studies to provide the basic data for constructing risk curves and benefit curves across the exposures to dietary and supplemental intakes of vitamin C, vitamin E, selenium, and β-carotene and other carotenoids. Studies should be designed to determine the relationship of nutrient intakes to validated biomarkers of oxidative stress. These studies should be followed by nested case-control studies to determine the relationship of the biomarkers of oxidative stress to chronic disease. Finally, full-scale intervention trials should be done to establish the preventive potential of a nutrient for chronic disease.
-
Investigations of gender specificity of the metabolism and requirements for vitamin C, vitamin E, selenium, and β-carotene and other carotenoids.
-
Studies to validate methods and possible models for estimating Dietary Reference Intakes (DRIs) in the absence of data for some life stage groups, such as children, pregnant and lactating women, and older adults.
-
Research to determine the interactions and possible synergisms of vitamin C, vitamin E, selenium, and β-carotene with each other, with other nutrients and food components, and with endogenous antioxidants. Multifactorial studies are needed to demonstrate in vivo actions as well as synergisms that have been shown to occur in vitro.
-
Studies to develop economical, sensitive, and specific methods to assess the associations of vitamin C, vitamin E, selenium, and β -carotene and other carotenoids with the causation, prevalence, prevention, and treatment of specific viral or other infections.
-
Investigations of the magnitude and role of genetic polymorphisms in the mechanisms of actions of vitamin C, vitamin E, selenium, and β-carotene and other carotenoids.
Source: https://www.nap.edu/read/9810/chapter/2

0 Komentar